End-to-End CQV & CSV Services for Regulated Industries
Metron Engineering delivers comprehensive Commissioning, Qualification, Validation (CQV) and Computer System Validation (CSV) services to ensure regulated facilities, equipment, utilities, and computerized systems operate reliably while meeting global compliance standards.
Our structured validation methodology supports organizations in achieving compliance with GMP, FDA, EU GMP, WHO, MHRA, and GAMP 5 guidelines, ensuring operational efficiency, product quality, and audit readiness.
Ensuring Compliance and Optimal Performance: Comprehensive Commissioning, Qualification & Validation (CQV) Services
In the highly regulated pharmaceutical, medical device, and biopharmaceutical industries, ensuring the quality, safety, and efficacy of products is paramount. This necessitates a robust and meticulously executed approach to Commissioning, Qualification & Validation (CQV). We provide comprehensive CQV services designed to optimize your systems, facilities, and equipment while guaranteeing full compliance with stringent industry regulations.
From the initial installation phase to Operational Qualification (OQ) and Performance Qualification (PQ), our expert team provides tailored solutions to meet your unique requirements. We understand the complexities of CQV and are committed to delivering efficient, reliable, and cost-effective services that minimize risk and maximize operational efficiency.
Our Comprehensive CQV Services Include:
- Commissioning: Ensuring your equipment, utilities, and facilities are properly installed, functionally tested, and ready for operation. This includes verification against design specifications and documentation of the commissioning process.
-
Qualification: Systematically demonstrating that equipment and systems operate correctly and consistently within predetermined parameters, meeting pre-defined acceptance criteria. This includes:
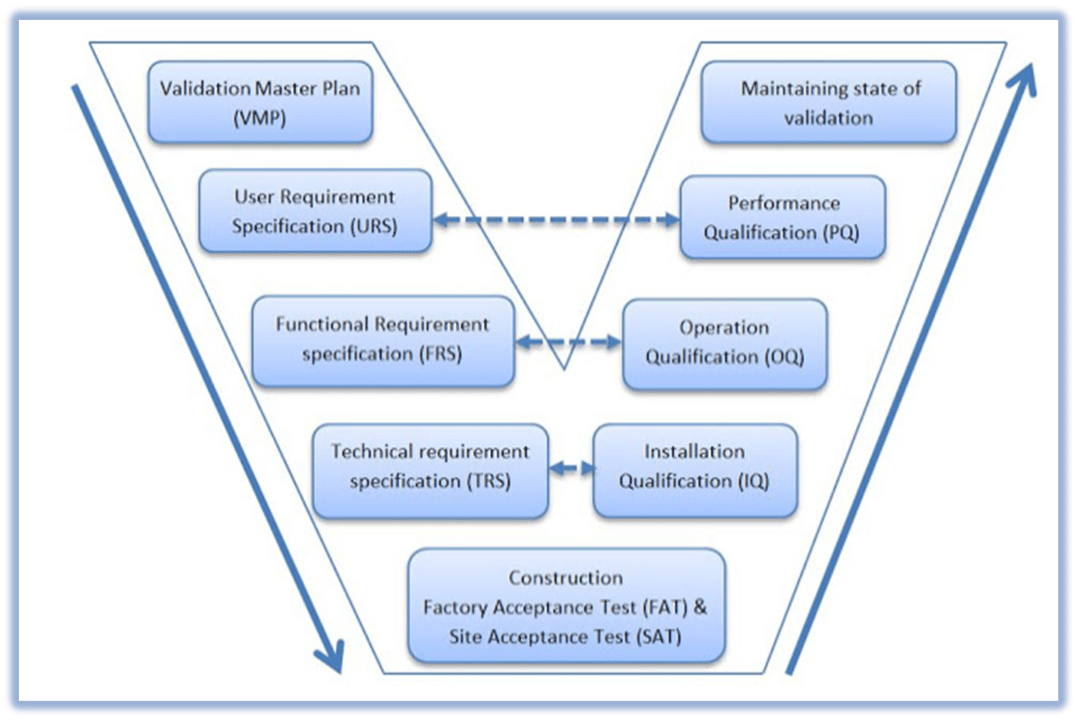
1. Installation Qualification (IQ): Verifying that equipment is installed correctly and in accordance with design specifications.
2. Operational Qualification (OQ): Demonstrating that equipment operates as intended throughout its operational range.
3. Performance Qualification (PQ): Confirming that the equipment, as part of a complete process, consistently performs as intended under routine operating conditions and produces acceptable product. - Validation: Establishing documented evidence that a process, system, or equipment consistently produces a product meeting pre-determined specifications and quality attributes. We meticulously document every step of the validation process, ensuring clear traceability and auditability.

- Risk Assessment & Mitigation: Identifying potential risks associated with your systems and processes and developing strategies to mitigate those risks, ensuring product quality and patient safety.
- Documentation & Reporting: Providing comprehensive documentation, including validation plans, protocols, reports, and Standard Operating Procedures (SOPs), ensuring full compliance with regulatory requirements.
- Gap Analysis & Remediation: Identifying gaps in your current CQV processes and providing solutions to address those gaps, ensuring full compliance.
- Training: Providing training to your personnel on proper equipment operation and maintenance procedures, ensuring consistent performance and compliance.

Why Choose Metron Engineering CQV Services?
- Expertise: Our team comprises highly experienced professionals with in-depth knowledge of pharmaceutical, medical device, and biopharmaceutical regulations, including FDA, EMA, and other global standards.
- Compliance: We are dedicated to ensuring your systems are fully compliant with all applicable regulations and guidelines.
- Efficiency: We streamline the CQV process, minimizing downtime and maximizing operational efficiency.
- Cost-Effectiveness: We offer competitive pricing and tailored solutions to meet your specific budget and requirements.
- Customization: We understand that every project is unique. We tailor our services to meet your specific needs and requirements.
By partnering with Metron Engineering Ltd for your Commissioning, Qualification & Validation needs, you can be confident that your systems are optimized for performance, compliant with industry regulations, and positioned for long-term success. Contact us today to discuss your specific requirements and learn how we can help you achieve your goals.
Computer System Validation (CSV) Services — Metron Engineering
Metron Engineering Ltd delivers expert Computer System Validation (CSV) services tailored for life sciences, pharmaceutical, medical device, and biopharmaceutical companies to ensure compliance with GxP regulatory requirements and audit readiness.
What We Offer:
- CSV for Equipment & Systems- We validate computerized systems that control, monitor, or record regulated equipment and processes to ensure they perform consistently and meet predefined specifications — supporting data integrity, reliability, and quality assurance throughout the equipment lifecycle.
- CSV for GxP Software Our services cover GxP‑relevant software applications and platforms, including systems used for manufacturing execution, quality management, laboratory operations, and data handling, ensuring they are compliant with regulations such as FDA 21 CFR Part 11 and GxP principles.

Core CSV Deliverables:
- Validation Planning & Strategy – Define scope, risk assessment, and lifecycle approach.
- Requirements & Specification Documentation – User Requirements (URS), Functional & Design Specs.
- Qualification Activities – IQ (Installation Qualification), OQ (Operational Qualification), PQ (Performance Qualification).
- Testing & Traceability – Risk‑based testing, traceability matrices, execution protocols.
- Compliance Documentation – Audit‑ready validation reports, change controls, SOPs.
- Lifecycle Support – Periodic review support and revalidation where needed.
Why Choose Metron Engineering CSV Services?
- Deep regulatory and engineering expertise tailored to GxP environments.
- Structured, risk‑based CSV approach aligned with industry standards such as GAMP 5 and GMP.
- Independent validation support from planning through execution and reporting.
Outcome:
Compliant, documented, and audit‑ready system validation that supports product quality, regulatory inspections, and operational confidence.
Industries We Serve:
- Pharmaceutical Manufacturing
- Biotechnology Companies
- Medical Device Manufacturers
- GMP Facilities
- Hospitals & Laboratories
- Food & Nutraceutical Industries
Get Expert CQV & CSV Support
Whether establishing a new facility or upgrading existing systems, our validation experts ensure smooth commissioning and sustainable compliance throughout the system lifecycle.
Contact our validation team today to discuss your project requirements.
FAQ – CQV & CSV Services
CQV is a structured process used in pharmaceutical and life sciences industries to verify that facilities, equipment, utilities, and systems are installed, operated, and performing according to regulatory and operational requirements.
CQV ensures compliance with GMP, FDA, EMA, and MHRA regulations while guaranteeing product quality, patient safety, and operational reliability.
1. IQ verifies correct installation
2. OQ confirms operational performance
3. PQ validates consistent performance under real conditions
CQV and CSV services are essential for:
1. Pharmaceutical manufacturing
2. Biopharmaceutical facilities
3. Medical device companies
4. GMP laboratories
5. Biotechnology organizations
CSV is the documented verification process confirming that computerized systems function accurately, securely, and in compliance with regulatory standards such as FDA 21 CFR Part 11.
Yes. Metron Engineering delivers commissioning, qualification, validation, and CSV services across the UK and European life sciences sector.
CQV provides documented evidence proving systems operate within validated parameters, ensuring audit readiness and minimizing compliance risks.
CQV demonstrates documented evidence that systems perform reliably, which is mandatory for GMP audits and regulatory approvals.
CSV activities typically follow GAMP 5 guidelines along with FDA, EU GMP Annex 11, and data integrity requirements.
Validation should begin during the design phase to reduce compliance risks and avoid costly rework later.

Our Clients
What They’re Saying








